Exploring Formations of Thio-Thiol and Keto-Enol Tautomers for Structural Analysis of 2-Thiouracil
DOI:
https://doi.org/10.22034/advjscieng21022111Keywords:
2-Thiouracil, Tautomer, Thio-thiol, Keto-enol, DFTAbstract
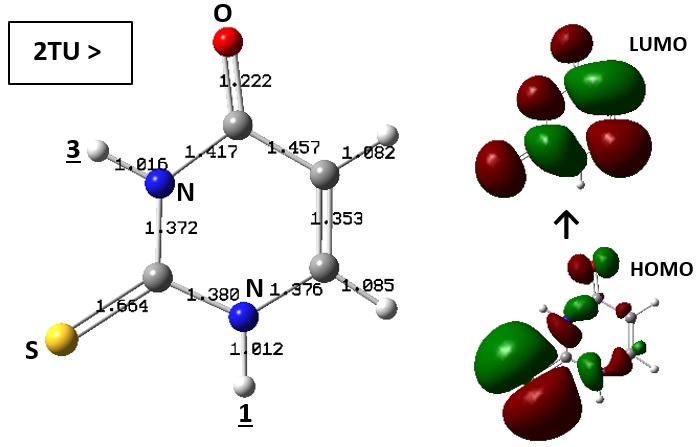
Formations of thio-thiol and keto-enol tautomers of 2-thiouracil (2TU) were investigated in this work for performing structural analysis by means of density functional theory (DFT) calculations. To this aim, existence of all possible structures were examined by movement of hydrogen atoms of amine groups to each of original thio and keto groups for formation of thiol and enol groups for new structures. Optimization calculations were performed to reach the minimum energy level for each investigated structure. The results showed that occurrence of such tautomerism processes could yield new features for the corresponding structures, in which variations were more or less significant in comparison with the original features. The important note is that formations of tautomers were possible meaning changes of expected activities regarding occurrence of such process in order to the concept of structure-activity relationship. Therefore, such computer-based works could provide information for examining such features in the tautomers of 2TU, as a compound related to pharmaceutical applications, to manage somehow the structural features for the specified purposes.
References
Yaraghi A, Ozkendir OM, Mirzaei M. DFT studies of 5-fluorouracil tautomers on a silicon graphene nanosheet. Superlattices and Microstructures. 2015;85:784-788.
Kouchaki A, Gülseren O, Hadipour N, Mirzaei M. Relaxations of fluorouracil tautomers by decorations of fullerene-like SiCs: DFT studies. Physics Letters A. 2016;380:2160-2166.
Ashjaee Y, Zandi H. Molecular analysis of 5-COR derivatives of uracil and evaluating their affinity against the MPro target of COVID-19. Advanced Journal of Science and Engineering. 2021;2:79-85.
Mirzaei M, Meskinfam M, Yousefi M. A cytosine-assisted carbon nanotubes junction: DFT studies. Superlattices and Microstructures. 2012;52:158-164.
Mirzaei M, Ravi S, Yousefi M. Modifying a graphene layer by a thymine or a uracil nucleobase: DFT studies. Superlattices and Microstructures. 2012;52:306-311.
Mirzaei M, Gulseren O. DFT studies of CNT–functionalized uracil-acetate hybrids. Physica E. 2015;73:105-109.
Sánchez-Rodríguez JA, Mohamadzade A, Mai S, Ashwood B, Pollum M, Marquetand P, González L, Crespo-Hernández CE, Ullrich S. 2-Thiouracil intersystem crossing photodynamics studied by wavelength-dependent photoelectron and transient absorption spectroscopies. Physical Chemistry Chemical Physics. 2017;19:19756-19766.
Lara NL, Silva Jr VA, Chiarini-Garcia H, Garcia SK, Debeljuk L, Hess RA, França LR. Hypothyroidism induced by postnatal PTU (6-n-propyl-2-thiouracil) treatment decreases Sertoli cell number and spermatogenic efficiency in sexually mature pigs. General and Comparative Endocrinology. 2020;299:113593.
Soleimani M, Mirzaei M, Mofid MR, Khodarahmi G, Rahimpour SF. Lactoperoxidase inhibition by tautomeric propylthiouracils. Asian Journal of Green Chemistry. 2020;4:1-10.
Hughes AM. Cretinism in rats induced by thiouracil. Endocrinology. 1944;34:69-76.
Hussein TS, Ahamad MR. Synthesis of N-glycosides analogues containing thiouracil unite and evaluated as antibacterial, antifungal and antioxidant active. Annals of the Romanian Society for Cell Biology. 2021;25:10915-10921.
Yaghoobi R, Mirzaei M. Computational analyses of cytidine and aza-cytidine molecular structures. Lab-in-Silico. 2020;1:21-26.
Zandi H, Harismah K. Computer-based tools for structural characterizations and activity specifications of natural products: a quick review. Lab-in-Silico. 2021;2:50-54.
Mirzaei M. 5–Fluorouracil: computational studies of tautomers and NMR properties. Turkish Computational and Theoretical Chemistry. 2017;1:27-34.
Frisch, M.; Trucks, G.; Schlegel, H.; Scuseria, G.; Robb, M.; Cheeseman, J.; Montgomery Jr, J.; Vreven, T.; Kudin, K.; Burant, J. Gaussian 09 D.01 Program. Gaussian. Inc.: Wallingford, CT, USA. 2009.
Fallahpour F, Ariaei S. Computational investigation of B6 particle for H2S capturing. Advanced Journal of Science and Engineering. 2021;2:31-35.
Taghipour M, Yousefi M, Fazaeli R, Darvishganji M. Gd impurity effect on the magnetic and electronic properties of hexagonal Sr ferrites: a case study by DFT. Chinese Physics B. 2020;29:077505.
Downloads
Published
How to Cite
Issue
Section
License
Copyright (c) 2021 Advanced Journal of Science and Engineering

This work is licensed under a Creative Commons Attribution 4.0 International License.
This work is licensed under a Creative Commons Attribution 4.0 International License (CC-BY 4.0).



