Synthesis of New Beta-Amidophosphonates and Theoretical Evaluation of Related Features
DOI:
https://doi.org/10.22034/advjscieng21022099Keywords:
Three-component reaction, Stable phosphorus ylides, Beta-amidophosphonates, DFTAbstract
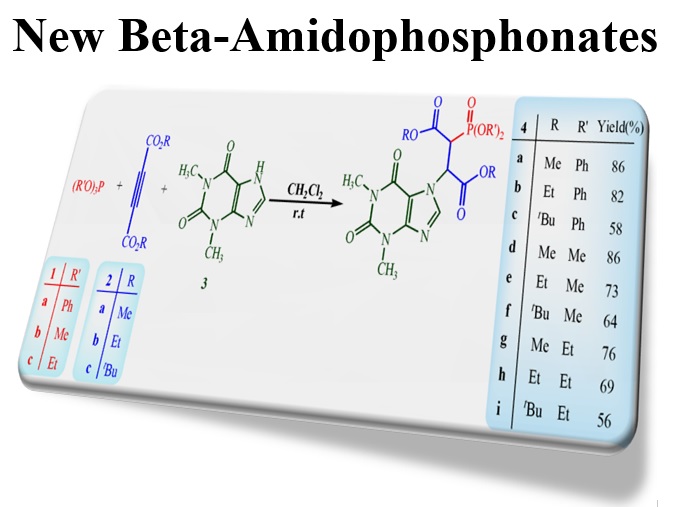
This work was done employing theoretical and experimental methodologies to show synthesis process of new compounds in addition to evaluating their features. In this case, phosphorus ylide intermediates were initially resulted from the three-component reaction between dialkyl acetylenedicarboxylate and theophiline in the presence of phosphits ((R’O)3P). Next, they were converted to stable diastereoisomeric beta-amidophosphonates. Density functional theory (DFT) calculations at the B3LYP/6-311+G* level of theory indicated that (2S, 3S) and/or (2R, 3R) diastereomers of phosphonates were about 18-28 kcal/mol more stable than (2R, 3S) and/or (2S, 3R) diastereomers. As a consequence, the targeted compounds were synthesized and they very confirmed by performing DFT calculations as an advantage of combinations of experimental and theoretical approaches for solving the problems in chemistry.
References
Noyori R. Asymetric catalysis in organic synthesis. John Wiley & Sons: NY. 1994.
Palacios F, Alonso C, de Los Santos JM. Synthesis of ?-Aminophosphonates and-Phosphinates. Chemical Reviews. 2005;105:899-932.
Engel R. Synthesis of carbon-phosphorus bond. CRC Press: Boca Raton, FL. 1988.
Kafarski P, LeJczak B. Biological activity of aminophosphonic acids. Phosphorus, Sulfur, and Silicon and the Related Elements. 1991;63:193-215.
Zare L, Nikpassand M. Multicomponent synthesis of dihydropyridines catalyzed by L-proline. Chinese Chemical Letters. 2011;22:531-534.
Allen JG, Atherton FR, Hall MJ, Hassall CH, Holmes SW, Lambert RW, Nisbet LJ, Ringrose PS. Phosphonopeptides, a new class of synthetic antibacterial agents. Nature. 1978;272:56-58.
Hakimelahi GH, Moosavi-Movahedi AA, Tsay SC, Tsai FY, Wright JD, Dudev T, Hakimelahi S, Lim C. Design, synthesis, and SAR of novel carbapenem antibiotics with high stability to xanthomonas maltophilia oxyiminocephalosporinase type II. Journal of Medicinal Chemistry. 2000;43:3632-3640.
Castelot?Deliencourt G, Roger E, Pannecoucke X, Quirion JC. Diastereoselective synthesis of chiral amidophosphonates by 1, 5?asymmetric induction. European Journal of Organic Chemistry. 2001;2001:3031-3038.
Dömling A, Ugi I. Multicomponent reactions with isocyanides. Angewandte Chemie International Edition. 2000;39:3168-3210.
Baharfar R, Baghbanian SM. Synthesis of novel uracil based 2, 5-diaminofurans using multi-component reactions. Chinese Chemical Letters. 2012;23:677-680.
Cherkasov RA, Pudovik MA. Heterophosphacyclanes in organic synthesis. Russian Chemical Reviews. 1994;63:1019-1045.
Yavari I, Bayat M. Synthesis of highly functionalized cyclobutene derivatives. Monatshefte für Chemie. 2003;134:1221-1227.
Yavari I, Kowsari E. Synthesis, spectral, and thermal properties of some phosphorus-containing 9, 10-anthraquininoid, thermally stable dyes. Dyes and Pigments. 2008;77:103-110.
Frisch MJ, Trucks GW, Schlegel HB, Scuseria GE, Robb MA, Cheeseman JR, et al. Gaussian 98 Program. Gaussian, Inc.: Pittsburgh, PA. 1998.
Karplus M. Vicinal proton coupling in nuclear magnetic resonance. Journal of the American Chemical Society. 1963;85:2870-2871.
Breitmaier E, Voelter W. Carbon-13 NMR spectroscopy. 3rd Ed. VCH: NY. 1990.
Norris WP, Henry RA. Cyanoguanyl Azide Chemistry1. The Journal of Organic Chemistry. 1964;29:650-660.
Francl MM, Pietro WJ, Hehre WJ, Binkley JS, Gordon MS, DeFrees DJ, Pople JA. Self?consistent molecular orbital methods. XXIII. A polarization?type basis set for second?row elements. The Journal of Chemical Physics. 1982;77:3654-3665.
Yavari I, Zabarjad-Shiraz N. Three-component reaction between triphenylphosphine, dialkyl acetylenedicarboxylates and urea or N-methylurea. Molecular Diversity. 2006;10:23-27.
Becke AD. Density-functional exchange-energy approximation with correct asymptotic behavior. Physical Review A. 1988;38:3098-3100.
Downloads
Additional Files
Published
How to Cite
Issue
Section
License
Copyright (c) 2021 Advanced Journal of Science and Engineering

This work is licensed under a Creative Commons Attribution 4.0 International License.
This work is licensed under a Creative Commons Attribution 4.0 International License (CC-BY 4.0).



