A DFT Study of H2 Molecule Adsorption at the Fullerene-Like Boron Carbide Nanocage
DOI:
https://doi.org/10.22034/advjscieng21021018Keywords:
Boron carbide, Fullerene-like, Nanocage, DFT, Gas sensorAbstract
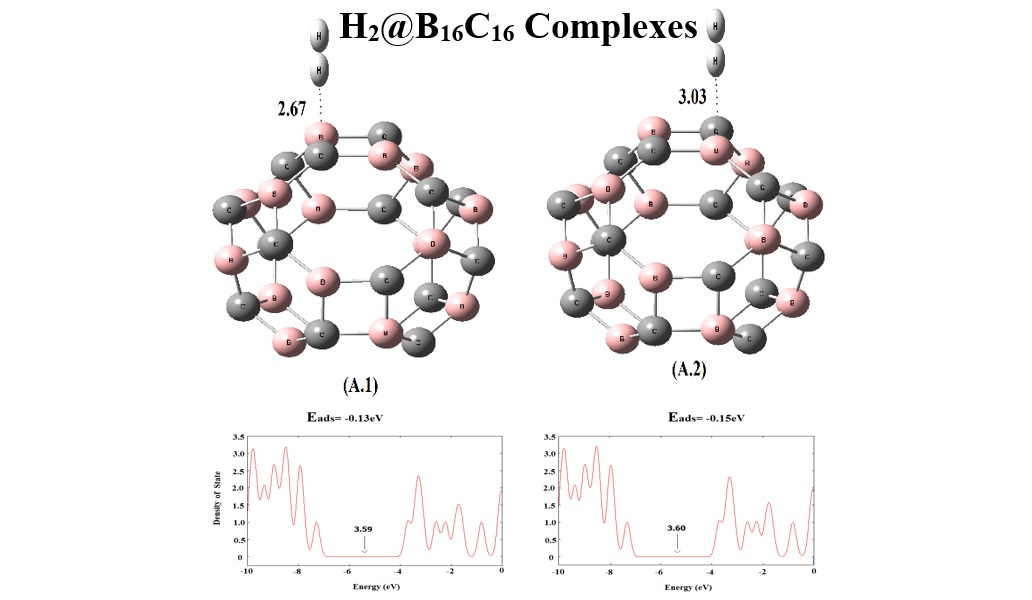
Equilibrium geometries, stabilities, and electronic properties of hydrogen (H2) molecule adsorption at the exterior surface of fullerene-like boron carbide (B16C16) were investigated through density functional theory (DFT) calculations. Indeed, sensor applications of such nanocage for H2 molecule were explored here. H2 molecule was physically adsorbed at the surface of B16C16 nanocage with adsorption energies of -0.13 and -0.15 eV. It was revealed that the electron transport through B16C16 was significantly increased in the presence of the H2 molecule due to the reduced frontier molecular orbitals energy gap. Based on the obtained results, it was expected that B16C16 nanocage could work as promising candidates in gas sensor devices of H2 molecule detections. The results also showed fairly short recovery time and high sensitivity benefits for B16C16 nanocage.
References
Kroto HW, Heath JR, O'Brien SC, Curl RF, Smalley RE. C60: buckminsterfullerene. Nature. 1985;318:162-163.
Contreras M, Avila D, Alvarez J, Rozas R. Exploring the structural and electronic properties of nitrogen-containing exohydrogenated carbon nanotubes: a quantum chemistry study. Structural Chemistry. 2010;21:573-581.
Tetasang S, Keawwangchai S, Wanno B, Ruangpornvisuti V. Quantum chemical investigation on structures of pyrrolic amides functionalized (5,5) single-walled carbon nanotube and their binding with halide ions. Structural Chemistry. 2012; 23:7-15.
Fallahpour F, Nouraliei M, Gorgani SS. Theoretical evaluation of a double–functional heterogeneous nano sensor. Applied Surface Science. 2016;366:545-551.
Ghamsari PA, Nouraliei M, Gorgani SS. DFT simulation towards evaluation the molecular structure and properties of the heterogeneous C16Mg8O8 nano-cage as selective nano-sensor for H2 and N2 gases. Journal of Molecular Graphics and Modelling. 2016;70:163-169.
Paine RT, NaruLa CK. Synthetic routes to boron nitride. Chemical Reviews. 1990;90:73-91.
Oku T, Kuno M, Kitahara H, Nartia I. A review of hydrogen storage systems based on boron and its compounds. International Journal of Inorganic Materials. 2001;3:597-612.
Gorgani SS, Nouraliei M, Gorgani SS. Heterogeneous C16Zn8O8 nanocluster as a selective CO/NO nanosensor: computational investigation. International Journal of Environmental Science and Technology. 2016;13:1573-1580.
Iranimanesh A, Yousefi M, Mirzaei M. DFT approach on SiC nanotube for NO2 gas pollutant removal. Lab-in-Silico. 2021;2:38-43.
Zhuiykov S, Wlodarski W, Li Y. Nanocrystalline V2O5-TiO2 thin-films for oxygen sensing prepared by sol-gel process. Sensors and Actuators B. 2001;77:484-490.
Chang H, Lee J, Lee S, Lee Y. Adsorption of NH3 and NO2 molecules on carbon nanotubes. Applied Physics Letters. 2001;79:3863-3865.
Cortight RD, Davada RR, Dumesic JA. Hydrogen from catalytic reforming of biomass-derived hydrocarbons in liquid water. Nature. 2002;418:964-967.
Alper J. Water splitting goes Au naturel. Science. 2003;299:1686-1687.
Schlapbach L, Züttel A. Hydrogen-storage materials for mobile applications. Nature. 2001;414:353-358.
Van den Berg AWC, Arean CO. Materials for hydrogen storage: current research trends and perspectives. Chemical Communications. 2008;6:668-681.
Sun Q, Jena P, Wang Q, Marquez M. First-principles study of hydrogen storage on Li12C60. Journal of the American Chemical Society. 2006;128:9741-9745.
Venkataramanan NS, Sahara R, Mizuseki H, Kawazoe Y. Hydrogen adsorption on lithium-functionalized calixarenes: a computational study. The Journal of Physical Chemistry C. 2008;112 ,19676-19679.
Woolf H, Brown I, Bowden M. Light metal hydrides - potential hydrogen storage materials. Current Applied Physics. 2008;8:459-462.
Fallahpour F, Gorgani SS, Nouraliei M. Boron carbide nanoclusters as H2 and N2 gases nanosensors: theoretical investigation. Indian Journal of Physics. 2016;90:931-936.
Moradi M, Nouraliei M, Moradi R. Theoretical study on the phenylpropanolamine drug interaction with the pristine, Si and Al doped C60 fullerenes. Physica E. 2017;87:186-191.
Dinadayalane TC, Leszczynski J. Stone-wales defects with two different orientations in (5,5) single-walled carbon nanotubes: a theoretical study. Chemical Physics Letters. 2007;434:86-91.
Dinadayalene TC, Murray JS, Concha MC, Politzer P, Leszczynski J. Reactivities of sites on (5,5) Single-walled carbon nanotubes with and without a stone-wales defect. Journal of Chemical Theory and Computations. 2010;6:1351-1357.
Rostami Z, Maskanati M, Khanahmadzadeh S, Dodangi M, Nouraliei M. Interaction of nitrotyrosine with aluminum nitride nanostructures: a density functional approach. Physica E. 2020;116:113735.
Mirzaei M. Making sense the ideas in silico. Lab-in-Silico. 2020;1:31-32.
Mirzaei M. A computational NMR study of boron phosphide nanotubes. Zeitschrift für Naturforschung A. 2010;65:844-848.
Aramideh M, Mirzaei M, Khodarahmi G, Gülseren O. DFT studies of graphene-functionalised derivatives of capecitabine. Zeitschrift fur Naturforschung A. 2017;72:1131-1138.
Mirzaei M, Gülseren O, Hadipour N. DFT explorations of quadrupole coupling constants for planar 5-fluorouracil pairs. Computational and Theoretical Chemistry. 2016;1090:67-73.
Mirzaei M. Science and engineering in silico. Advanced Journal of Science and Engineering. 2020;1:1-2.
Mirzaei M, Hadipour NL. An investigation of hydrogen-bonding effects on the nitrogen and hydrogen electric field gradient and chemical shielding tensors in the 9-methyladenine real crystalline structure: a density functional theory study. The Journal of Physical Chemistry A. 2006;110:4833-2838.
Schmidt MW, Baldridge KK, Boatz JA, Elbert ST, Gordon MS, Jensen JH, Koseki S, Matsunaga N, Nguyen KA, Su S, Windus TL. General atomic and molecular electronic structure system. Journal of Computational Chemistry. 1993;14:1347-1363.
Downloads
Published
How to Cite
Issue
Section
License
Copyright (c) 2021 Advanced Journal of Science and Engineering

This work is licensed under a Creative Commons Attribution 4.0 International License.
This work is licensed under a Creative Commons Attribution 4.0 International License (CC-BY 4.0).



