DFT Approach on Arsine and Phosphine Gases Adsorption at the Surface of B16C16 Nanocluster
DOI:
https://doi.org/10.22034/labinsilico20012044Keywords:
Arsine, Phosphine, Adsorption, Nanocluster, DFTAbstract
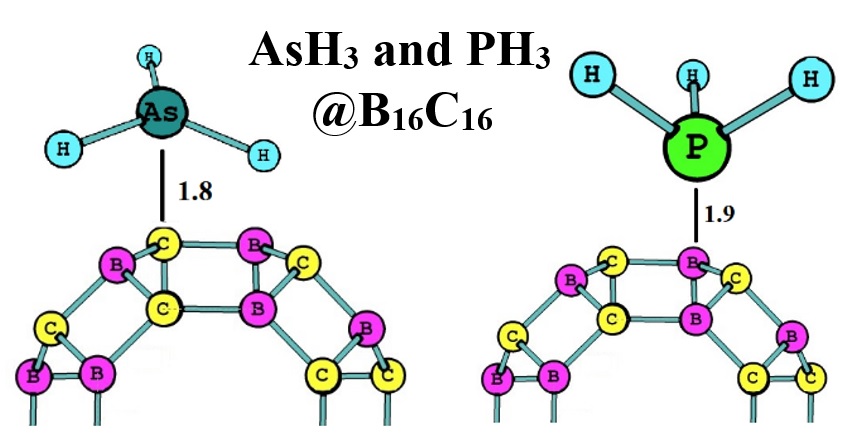
In this study, interactions of arsine (AsH3) and phosphine (PH3) gas molecules with B16C16 nanocluster were investigated using M06-2X/6-311G(d,p) density functional theory (DFT) method. Results showed that the electron density of adsorbed molecules could play an important role in adsorption of AsH3 and PH3 at the surface of B16C16 (on the top of B and C atoms of the cluster). Calculated amounts of adsorption energy were -3.50 and -2.90 eV for AsH3 and PH3, respectively. It revealed that the B16C16 nanocluster could work selectively for adsorbing each of AsH3 and PH3 gas molecules. The obtained achievement was approved regarding the calculated properties of molecular orbital energies, band gap, and charge transfer for the optimized systems.
References
Iijima S. Synthetic nano-scale fibrous matrix. Nature. 1991;56:354-358.
Faramarzi R, Falahati M, Mirzaei M. Interactions of fluorouracil by CNT and BNNT: DFT analyses. Adv J Sci Eng. 2020;1:62-66.
Mirzaei M, Hadipour NL, Seif A, Giahi M. Density functional study of zigzag BN nanotubes with equivalent ends. Physica E. 2008;40:3060-3063.
Nouri A, Mirzaei M. DFT calculations of B-11 and N-15 NMR parameters in BN nanocone. J Mol Struct THEOCHEM. 2009;913:207-209.
Mirzaei M, Hadipour NL. Density functional calculations of 14N and 11B NQR parameters in the H-capped (6, 0) and (4, 4) single-walled BN nanotubes. Physica E. 2008;40:800-804.
Mirzaei M, Mirzaei M. A computational study of oxygen-termination of a (6, 0) boron nitride nanotube. Monatsh Chem. 2010;141:491-494.
Mirzaei M, Mirzaei M. Electronic structure of sulfur terminated zigzag boron nitride nanotube: A computational study. Solid State Sci. 2010;12:1337-1340.
Mirzaei M, Meskinfam M. Computational studies of effects of tubular lengths on the NMR properties of pristine and carbon decorated boron phosphide nanotubes. Solid State Sci. 2011;13:1926-1930.
Mirzaei M, Mirzaei M. The B-doped SiC nanotubes: A computational study. J Mol Struct THEOCHEM. 2010;953:134-138.
Mirzaei M, Mirzaei M. The C-doped AlP nanotubes: A computational study. Solid State Sci. 2011;13:244-250.
Mirzaei M. The NMR parameters of the SiC-doped BN nanotubes: a DFT study. Physica E. 2010;42:1954-1957.
Mirzaei M. Effects of carbon nanotubes on properties of the fluorouracil anticancer drug: DFT studies of a CNT-fluorouracil compound. Int J Nano Dimens. 2013;3:175-179.
Mirzaei M, Yousefi M. Computational studies of the purine-functionalized graphene sheets. Superlatt Microstruct. 2012;52:612-617.
Mirzaei M, Kalhor HR, Hadipour NL. Covalent hybridization of CNT by thymine and uracil: A computational study. J Mol Model. 2011;17:695-699.
Harismah K, Ozkendir OM, Mirzaei M. Lithium adsorption at the C20 fullerene-like cage: DFT approach. Adv J Sci Eng. 2020;1:74-79.
Mirzaei M. Nanotechnology for science and engineering. Adv J Sci Eng. 2020;1:67-68.
Tahmasebi E, Shakerzadeh E. Potential application of B40 fullerene as an innovative anode material for Ca-ion batteries: In silico investigation. Lab-in-Silico. 2020;1:16-20.
Gupta VK, Saleh TA. Sorption of pollutants by porous carbon, carbon nanotubes and fullerene-An overview. Environ Sci Pollut Res. 2013;20:2828-2843.
Rikame SS, Mungray AA, Mungray AK. Synthesis, characterization and application of phosphorylated fullerene/sulfonated polyvinyl alcohol (PFSP) composite cation exchange membrane for copper removal. Separat Purif Technol. 2017;177:29-39.
Khan AA, Ahmad I, Ahmad R. Influence of electric field on CO2 removal by P-doped C60-fullerene: A DFT study. Chem Phys Lett. 2020;742:137155.
Ong SL, Hu JY, Biryulin YF, Polotskaya GA. Fullerene?containing polymer membranes for rejection of estrogenic compounds in water. Full Nano Carbon Nonstruct. 2006;14:463-466.
Jun LY, Mubarak NM, Yee MJ, Yon LS, Bing CH, Khalid M, Abdullah EC. An overview of functionalised carbon nanomaterial for organic pollutant removal. J Indust Eng Chem. 2018;67:175-186.
Baniasadi R, Harismah K, Sadeghi M, Mirzaei M. Adsorption of vitamin C on a fullerene surface: DFT studies. J Nanoanalys. 2017;4:1-7.
Kouchaki A, Gülseren O, Hadipour N, Mirzaei M. Relaxations of fluorouracil tautomers by decorations of fullerene-like SiCs: DFT studies. Phys Lett A. 2016;380:2160-2166.
Harismah K, Mirzaei M, Ghasemi N, Nejati M. Non-covalent functionalisation of C30 fullerene by pyrrole-n-carboxylic acid (n= 2, 3): Density functional theory studies. Z Naturforsch A. 2017;73:51-56.
Vergara Reyes HN, Chigo Anota E, Castro M. C60-like boron carbide and carbon nitride fullerenes: stability and electronic properties obtained by DFT methods. Full Nano Carbon Nanostruct. 2018;26:52-60.
Mahabal MS, Deshpande MD, Hussain T, Ahuja R. Sensing characteristics of phosphorene monolayers toward PH3 and AsH3 gases upon the introduction of vacancy defects. J Phys Chem C. 2016;120:20428-20436.
Alexeev Y, P Mazanetz M, Ichihara O, G Fedorov D. GAMESS as a free quantum-mechanical platform for drug research. Curr Topics Med Chem. 2012;12:2013-2033.
Peralta-Inga Z, Lane P, Murray JS, Boyd S, Grice ME, O'Connor CJ, Politzer P. Characterization of surface electrostatic potentials of some (5, 5) and (n, 1) carbon and boron/nitrogen model nanotubes. Nano Lett. 2003;3:21-28.
Fallahpour F, Nouraliei M, Gorgani SS. Theoretical evaluation of a double–functional heterogeneous nano-sensor. Appl Surf Sci. 2016;366:545-551.
Politzer P, Lane P, Murray JS, Concha MC. Comparative analysis of surface electrostatic potentials of carbon, boron/nitrogen and carbon/boron/nitrogen model nanotubes. J Mol Model. 2005;11:1-7.
Ghamsari PA, Nouraliei M, Gorgani SS. DFT simulation towards evaluation the molecular structure and properties of the heterogeneous C16Mg8O8 nano–cage as selective nano–sensor for H2 and N2 gases. J Mol Graph Model. 2016;70:163-169.
Saedi L, Javanshir Z, Khanahmadzadeh S, Maskanati M, Nouraliei M. Determination of H2S, COS, CS2 and SO2 by an aluminium nitride nanocluster: DFT studies. Mol Phys. 2020;118:e1658909.
Rostami Z, Maskanati M, Khanahmadzadeh S, Dodangi M, Nouraliei M. Interaction of nitrotyrosine with aluminum nitride nanostructures: A density functional approach. Physica E. 2020;116:113735.
Mirzaei M, Hadipour NL, Abolhassani MR. Influence of C-doping on the B-11 and N-14 quadrupole coupling constants in boron-nitride nanotubes: A DFT study. Z Naturforsch A. 2007;62:56-60.
Mirzaei M. Calculation of chemical shielding in C-doped zigzag BN nanotubes. Monatsh Chem. 2009;140:1275-1278.
Mirzaei M. A computational NMR study of boron phosphide nanotubes. Z Naturforsch A. 2010;65:844-848.
Gunaydin S, Alcan V, Mirzaei M, Ozkendir OM. Electronic structure study of Fe substituted RuO2 semiconductor. Lab-in-Silico. 2020;1:7-10.
Samadi Z, Mirzaei M, Hadipour NL, Khorami SA. Density functional calculations of oxygen, nitrogen and hydrogen electric field gradient and chemical shielding tensors to study hydrogen bonding properties of peptide group (OC–NH) in crystalline acetamide. J Mol Graph Model. 2008;26:977-981.
Mirzaei M, Hadipour NL, Ahmadi K. Investigation of C–H…OC and N–H…OC hydrogen-bonding interactions in crystalline thymine by DFT calculations of O-17, N-14 and H-2 NQR parameters. Biophys Chem. 2007;125:411-415.
Mirzaei M, Gülseren O, Hadipour N. DFT explorations of quadrupole coupling constants for planar 5-fluorouracil pairs. Comput Theor Chem. 2016;1090:67-73.
Yaghoobi R, Mirzaei M. Computational analyses of cytidine and aza-cytidine molecular structures. Lab-in-Silico. 2020;1:21-25.
Partovi T, Mirzaei M, Hadipour NL. The C–H..O Hydrogen bonding effects on the 17O electric field gradient and chemical shielding tensors in crystalline 1-methyluracil: A DFT study. Z Naturforsch A. 2006;61:383-388.
Ozkendir OM. Temperature dependent XAFS study of CrFe2O4. Lab-in-Silico. 2020;1:33-37.
Agil H, Akduran N. Structural, electrical and magnetic properties of FeO added GdBaCuO superconductors. Adv J Sci Eng. 2020;1:122-127.
Mirzaei M. Science and engineering in silico. Adv J Sci Eng. 2020;1:1-2.
Downloads
Published
How to Cite
Issue
Section
License
Copyright (c) 2020 Lab-in-Silico

This work is licensed under a Creative Commons Attribution 4.0 International License.




