Potential Application of B40 Fullerene as an Innovative Anode Material for Ca-ion Batteries: In Silico Investigation
DOI:
https://doi.org/10.22034/labinsilico20011016Keywords:
B40 fullerene, Open-circuit voltage, Ca-ion batteries, DFT calculationsAbstract
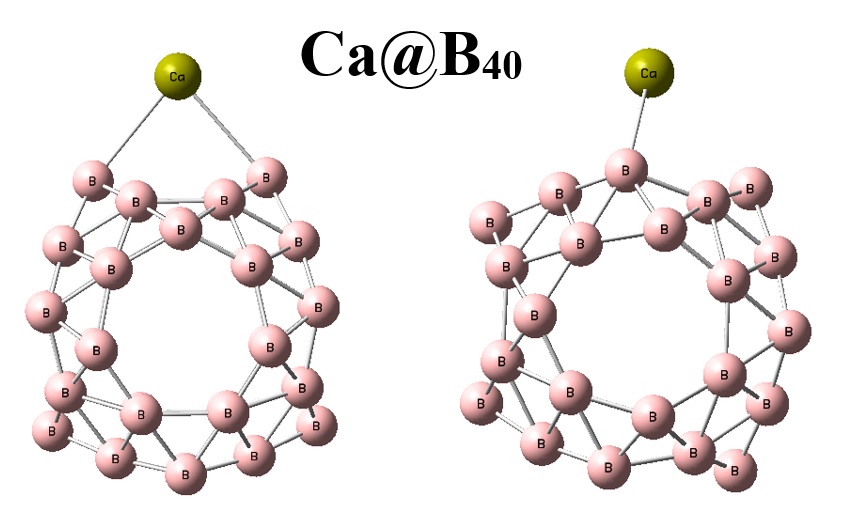
Density functional theory (DFT) calculations were performed using the PBE0-D3 functional and the 6-31+G(d) basis set to determine the potential application of recent experimentally observed B40 fullerene for the anode electrode for Ca-ion batteries (CIBs) in silico. The interactions of both Ca and Ca2+ with the B40 fullerene were investigated for the purpose. Based on the calculated results, the bare B40 fullerene have been seen as a promising anode material with remarkable average open-circuit voltage of 4.52 V and storage capacity of 744 mAhg–1. The obtained results of this study might open new windows for designing such promising boron-based anode materials for CIBs, which is an advantage of computer-based works for novel technologies. Such novel types of batteries are very much important to be developed for applications in high level technologies and industries.
References
Dunn B, Kamath H, Tarascon JM. Electrical energy storage for the grid: A battery of choices. Science. 2011;334:928-935.
Soloveichik GL. Battery technologies for large-scale stationary energy storage. Ann Rev Chem Biomol Engin. 2011;2:503-527.
Cheng F, Liang J, Tao Z, Chen J. Functional materials for rechargeable batteries. Adv Mater. 2011;23:1695-1715.
Liu C, Li F, Lai-Peng M, Cheng HM. Advanced materials for energy storage. Adv Mater. 2010;22(8):28-62.
Duffner F, Wentker M, Greenwood M, Leker J. Battery cost modeling: A review and directions for future research. Renew Sustain Energy Rev. 2020;127:109872.
Raccichini R, Varzi A, Passerini S, Scrosati B. The role of graphene for electrochemical energy storage. Nat Mater. 2015;14:271-279.
Chan CK, Peng H, Liu G, McIlwrath K, Zhang XF, Huggins RA, et al. High-performance lithium battery anodes using silicon nanowires. Nat Nanotechnol. 2008;3:31-35.
Ma C, Shao X, Cao D. Nitrogen-doped graphene nanosheets as anode materials for lithium ion batteries: A first-principles study. J Mater Chem. 2012;22:8911-5.
Goodenough JB, Park KS. The Li-ion rechargeable battery: A perspective. J Am Chem Soc. 2013;135:1167-1176.
Opitz A, Badami P, Shen L, Vignarooban K, Kannan AM. Can Li-Ion batteries be the panacea for automotive applications? Renew Sustain Energy Rev. 2017;68:685-92.
O’Heir J. Building better batteries. Mech Eng. 2017;139:10-11.
Wang R, Cui W, Chu F, Wu F. Lithium metal anodes: Present and future. J Energy Chem. 2020;48:145-159.
Mukherjee S, Singh G. Two-dimensional anode materials for non-lithium metal-ion batteries. ACS Appl Energy Mater. 2019;2:932-955.
Wang Y, Chen R, Chen T, Lv H, Zhu G, Ma L, et al. Emerging non-lithium ion batteries. Energy Storage Mater. 2016;4:103-129.
Chen X, Wang S, Wang H. High performance hybrid Mg-Li ion batteries with conversion cathodes for low cost energy storage. Electrochim Acta. 2018;265:175-183.
Nejati K, Hosseinian A, Bekhradnia A, Vessally E, Edjlali L. Na-ion batteries based on the inorganic BN nanocluster anodes: DFT studies. J Mol Graph Model. 2017;74:1-7.
Benzidi H, Lakhal M, Garara M, Abdellaoui M, Benyoussef A, El Kenz A, et al. Arsenene monolayer as an outstanding anode material for (Li/Na/Mg)-ion batteries: Density functional theory. Phys Chem Chem Phys. 2019;21:19951-19962.
Kosar N, Asgar M, Ayub K, Mahmood T. Halides encapsulation in aluminum/boron phosphide nanoclusters: An effective strategy for high cell voltage in Na-ion battery. Mater Sci Semicond Process. 2019;97:71-79.
Tao ZL, Xu LN, Gou XL, Chen J, Yuan HT. TiS2 nanotubes as the cathode materials of Mg-ion batteries. Chem Commun. 2004;10:2080-2081.
Aslanzadeh SA. A computational study on the potential application of zigzag carbon nanotubes in Mg-ion batteries. Struct Chem. 2020; in press.
Liu Y, Sun Z, Tan K, Denis DiK, Sun J, Liang L, et al. Recent progress in flexible non-lithium based rechargeable batteries. J Mater Chem A. 2019;7:4353-4382.
Gummow RJ, Vamvounis G, Kannan MB, He Y. Calcium-ion batteries: Current state-of-the-art and future perspectives. Adv Mater. 2018;30:1-14.
Park J, Xu Z, Yoon G, Park SK, Wang J, Hyun H. Stable and high-power calcium-ion batteries enabled by calcium intercalation into graphite. Adv Mater. 2020;32:1904411.
Romanescu C, Galeev TR, Sergeeva AP, Li W-L, Wang L-S, Boldyrev AI. Experimental and computational evidence of octa- and nona-coordinated planar iron-doped boron clusters: Fe@B8? and Fe@B9?. J Organomet Chem. 2012;721:148-154.
Jian T, Chen X, Li SD, Boldyrev AI, Li J, Wang LS. Probing the structures and bonding of size-selected boron and doped-boron clusters. Chem Soc Rev. 2019;48:3550-3591.
Popov IA, Jian T, Lopez G V., Boldyrev AI, Wang LS. Cobalt-centred boron molecular drums with the highest coordination number in the CoB16- cluster. Nat Commun. 2015;6:1-7.
Zubarev DY, Boldyrev AI. Comprehensive analysis of boron clusters. J Comput Chem. 2007;28:251-268.
Pham HT, Duong L V., Nguyen MT. Electronic structure and chemical bonding in the double ring tubular boron clusters. J Phys Chem C. 2014;118:24181-24187.
Pham HT, Pham-Ho MP, Nguyen MT. Impressive capacity of the B7? and V2B7 clusters for CO2 capture. Chem Phys Lett. 2019;728:186-194.
Zhai HJ, Zhao YF, Li WL, Chen Q, Bai H, Hu HS, et al. Observation of an all-boron fullerene. Nat Chem. 2014;6:727-731.
Yang Y, Zhang Z, Penev ES, Yakobson BI. B40 cluster stability, reactivity, and its planar structural precursor. Nanoscale. 2017;9:1805-1810.
Maniei Z, Shakerzadeh E, Mahdavifar Z. Theoretical approach into potential possibility of efficient NO2 detection via B40 and Li@B40 fullerenes. Chem Phys Lett. 2018;691:360-365.
Fa W, Chen S, Pande S, Zeng XC. Stability of Metal-Encapsulating Boron Fullerene B40. J Phys Chem A. 2015;119:11208-11214.
Bai H, Chen Q, Zhai HJ, Li SD. Endohedral and exohedral metalloborospherenes: M@B40 (M= Ca, Sr) and M@B40 (M= Be, Mg). Angew Chem. 2015;54:941-945.
Moradi M, Bagheri Z, Bodaghi A. Li interactions with the B40 fullerene and its application in Li-ion batteries: DFT studies. Physica E. 2017;89:148-54.
Mirzaei M. Nanotechnology for science and engineering. Adv J Sci Eng. 2020;1:67-68.
Faramarzi R, Falahati M, Mirzaei M. Interactions of fluorouracil by CNT and BNNT: DFT analyses. Adv J Sci Eng. 2020;1:62-66.
Ulfat I, Ahmed SA, Iqbal SMZ, Kamaluddin S, Mehmood Z, Kanwal S. Synthesis and characterization of gold nanoparticles. Adv J Sci Eng. 2020;1:48-51.
Ozkendir OM. Electronic structure study of Sn-substituted InP semiconductor. Adv J Sci Eng. 2020;1:7-11
Soleimanimehr H, Mirzaei M, Ghani M, Sattari F, Forouzan Najafabadi A. Micro-grooving of aluminum, titanium and magnesium alloys by acidithiobacillus ferrooxidans bacteria. Adv J Sci Eng. 2020;1:16-19.
Mirzaei M. The NMR parameters of the SiC-doped BN nanotubes: a DFT study. Physica E. 2010;42:1954-1957.
Mirzaei M. Lab-in-Silico: An international journal. Lab-in-Silico. 2020;1:1-2.
Harismah K, Mirzaei M. In silico interactions of steviol with monoamine oxidase enzymes. Lab-in-Silico. 2020;1:3-6.
Gunaydin S, Alcan V, Mirzaei M, Ozkendir OM. Electronic structure study of Fe substituted RuO2 semiconductor. Lab-in-Silico. 2020;1:7-10.
Harismah K, Mirzaei M. Steviol and iso-steviol vs. cyclooxygenase enzymes: In silico approach. Lab-in-Silico. 2020;1:11-15.
Tian Y, Wei D, Jin Y, Barroso J, Lu C, Merino G. Exhaustive exploration of MgBn (n= 10-20) clusters and their anions. Phys Chem Chem Phys. 2019;21:6935-6941.
Frisch MJ, Trucks GW, Schlegel HB, Scuseria GE, Robb MA, Cheeseman JR, et al. Gaussian 09 A.01. Gaussian Inc., Wallingford; 2013.
Downloads
Published
How to Cite
Issue
Section
License
Copyright (c) 2020 Lab-in-Silico

This work is licensed under a Creative Commons Attribution 4.0 International License.




